- Atoms To Mass In Grams Converter Calculator Calculator
- Atoms To Mass In Grams Converting Calculator
- Atoms To Mass In Grams
- Mass To Atoms Calculator
- Moles To Atoms Calculator
- How Do You Convert Atoms To Mass In Grams
To convert mass to atoms:
Mole unit conversion between moles and atoms, atoms to moles conversion in batch, mol atoms conversion chart. Note: Fill in one box to get results in the other box by clicking 'Calculate' button. Data should be separated in coma (,), space ( ), tab, or in separated lines. Begin: Step: » Moles Conversions. • Flow Mass • Force. What is the formula for converting atoms to grams? Here is a website in related links that will give a conversion of volume to mass of various cooking ingredients. How to Convert Grams to Curies. WikiHow is a wiki similar to Wikipedia, which means that many of our articles are written collaboratively. This can be done by dividing the amount (in grams) by the atomic mass of the material. Calculate the number of atoms in the given amount of the radioactive material. Moles to Grams Calculator » Common Compounds List » Chemical Equation Balancer » Complete List of Acids » Complete List of Bases » Molar to Mass Concentration Converter » Molar Mass Calculator » Cations, Anions List » Dilution Calculator » Molarity Calculator » Compound Prefixes » Water Insoluble Compounds » Compound Quiz. To convert mass to atoms: Find the atomic mass of the element in the substance. You can find atomic masses on the periodic table. Lithium's atomic mass is 6.9 grams (round if you need to) Then. Grams to Moles Calculator. Mole is a standard measurement of amount which is used to measure the number atoms (or) molecules. 1 mole of something is equal to 6.0221415x10 23 of it. This online unit converter will help you to convert the grams of a molecule to the number of moles based on the weight of given chemical equation / formula.
Find the atomic mass of the element in the substance. You canfind atomic masses on the periodic table. Ex. Lithium's atomic massis 6.9 grams (round if you need to)
Then find the mass of the substance in grams. Ex. you have 18.2grams of a sample of Lithium.
The mass of the sample is multiplied by 6.02 * 1023 anddivided by the atomic mass.
Ex.
mass of sample in grams * (6.02 * 1023 atoms) / (atomic mass) =# atoms in grams
18.2 grams * (6.02 * 1023 atoms) / (6.9 grams) = 1.59*1024atoms
The number 6.02 * 1023 is Avogadro's Constant which is theamount of atoms (or molecules) in one mole.
How to convert grams to atoms?
To convert grams into atoms, you have to convert them into moles first. Get the molar mass and multiply it by the number of moles to get the atoms.
What do atoms convert mass into?
In a nuclear reaction, the mass of atoms is converted into energy according to Einstein's formula E = mc2.
What do atoms convert mass in to?
The mass can be converted in energy: the equation of Einstein is: E= mc2.
How do you convert mass to moles and moles to mass?
To convert mass to moles, you use the equation n= m/M n is moles, m is mass, M= Molar mass, which is the mass of the atoms in the formula or substance.
What is the mass of atoms in grams of 1.02X10 to the 24 atoms Mn?
1 mole manganese = 54.938049g 1 mole Mn atoms = 6.022 x 1023 atoms Convert atoms Mn to moles Mn. 1.02 x 1024 atoms Mn x 1mol/6.022 x 1023 atoms = 1.69mol Mn Convert moles Mn to mass in grams Mn. 1.69mol Mn x 54.938049g/mol = 92.8g
How do you convert moles of atoms to grams?
Multiply number of moles by the molecular mass of the element or compound
What is the mass of 1.505 x 10²³ carbon atoms?
To convert atoms to grams, you need to take the number of atoms, divide it by Avogadro's Constant, then multiply it by the atomic mass. Atoms ÷ (6.02 × 1023) × Atomic mass = Mass in grams 1.505 × 1023 ÷ (6.02 × 1023) × 12.0 = 3.00 grams Carbon
Grams of mercury to Atoms of mercury?
Converting Grams into Atoms You must first convert grams into moles, then you can convert into atoms. Converting grams into moles: Grams of Mercury / 200.59 (Mass of 1 Mole of Mercury) Converting moles into atoms: Answer to the above * 6.02 x 1023 (Avogadro's Constant) Avogadro's Constant / Mole = 6.022 x 1023 Atomic Mass of Mercury = 200.59
How do you convert from grams to atoms and from mass to atoms?
1 atomgram of a chemical element has 6,02214129(27)×1023 atoms. 1 atomgram=atomic weight of a chemical element exprimed in grams.
What relationships are needed to convert 1.5 grams of carbon to the number of carbon atoms?
How do you convert the mass of a substance to the number of moles in a substance?
Atoms To Mass In Grams Converter Calculator Calculator
You must first calculate the molar mass of the substance. To do so, you add up the molar masses of all the elements that make up the compound, multiplied by the number of atoms of that element in one molecule of the substance. For example, AgNO3 has a molar mass of about 169.8 amu. In one molecule of AgNo3, there is one atom of Silver (molar mass 107.8), one atom of Nitrogen (molar mass 14)…
How do you convert molecules to atoms with avogadro's number?
In order to find the number of atoms in a molecule you must convert the molecules mass to molar mass, then convert to number of atoms by use of Avagadros number. Take for instance 5grams of Table salt (NaCl). First we need to get Molar mass in order to determine number of atoms. 1 mole of NaCl is molar mass of one mole of Na (23) + molar mass of one mole of Cl (35)…
What are the steps needed to convert the number of atoms to the mass of an elementt?
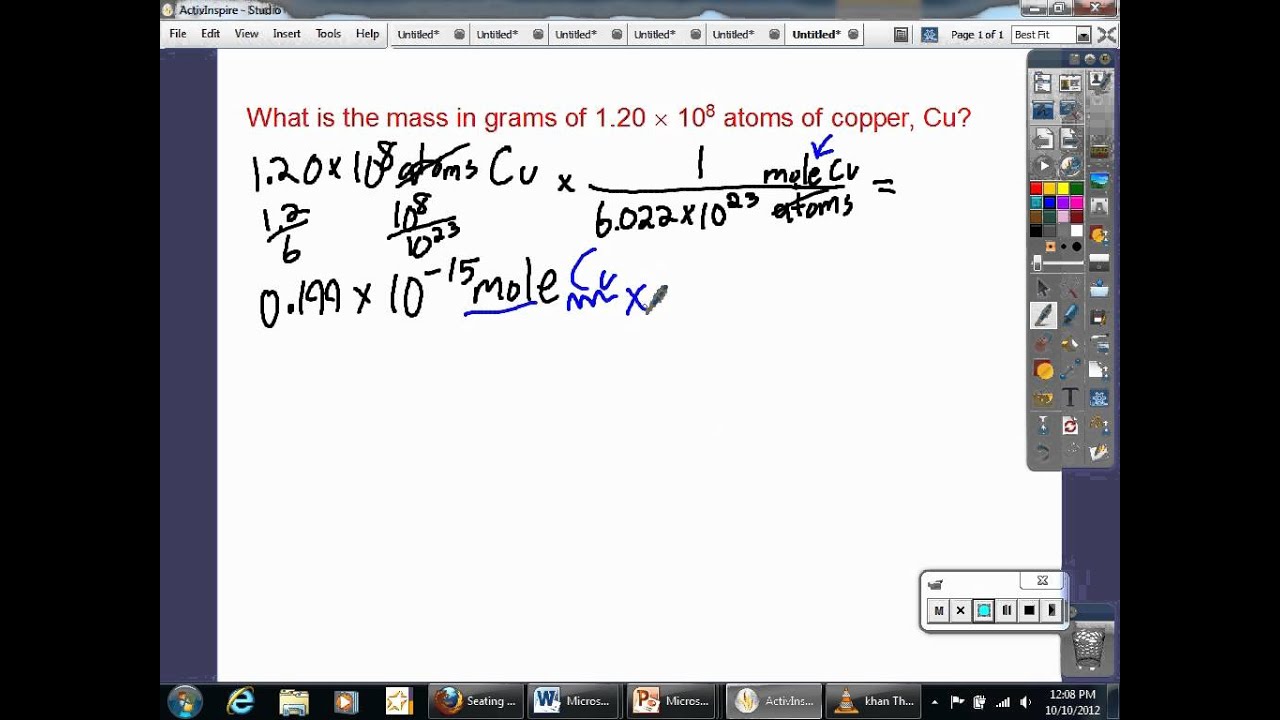
1 mole = 6.022 x 1023 atoms = molar mass in g/mole ?atoms x 1 mole/6.022 x 1023 atoms x molar mass = mass in grams For example: What is the mass of 2.00 x 1024 atoms of carbon? Answer: 1 mole C = 6.022 x 1023 atoms C = 12.0107g/mole C 2.00 x 1024 atoms C x 1 mole C/6.022 x 1023 atoms C x 12.0107g/mole C = 39.9g C
How do you find the mass of a sample of 1.72 x 1023 atoms of potassium?
1 mole K atoms = 39.0983g K (atomic weight in grams) 1 mole K atoms = 6.022 x 1023 atoms K (Avogadro's number) Convert known atoms to moles. 1.72 x 1023 atoms K x (1mol K/6.022 x 1023 atoms K) = 0.286mol K Convert moles to mass in grams. 0.286mol K x (39.0983g K/1mol K) = 11.2g K
What is the mass in grams of 2.01 x 1022 atoms of sulfur?
1 mole S atoms = 32.065 g S (atomic weight in grams) 1 mole S atoms = 6.022 x 1023 atoms S (Avogadro's number) Convert atoms to moles. 2.01 x 1022 atoms S x (1mol S/6.022 x 1023 atoms S) = 0.0334mol S Convert moles to mass in grams. 0.334mol S x (32.065g S/1mol S) = 10.7g S
Explain the steps needed to convert the mass of an element to the number of atoms of the element?
1. Find the atomic mass of the element in atomic mass units. 2. Divide the mass of the element by its atomic mass. 3. Multiply the result by Avogadro's Number.
How do you convert atoms to molecules and molecules to atoms?
In general, by chemical reaction to convert atoms to molecules and chemical, electrolytic, and/or photolytic dissociation to convert molecules to atoms. The details depend on the specific elements and compounds.
Do atoms have mass?
How do you convert from molecular mass to grams per mole?
Relative molecular mass is obtained by summing up the atomic masses of atoms in the formula. The gram molecular mass denotes the mass of a mole of the substance in grams. Both of them are same in number.
How do you convert 2.11x10 to the 24 atoms of copper to mass in grams?
2.11x10^24 atoms... Divide by Avogadro's Constant (6.02x10^23 particles/mole) Gives you moles of copper... Multiply by the molar mass of copper. (?? g/mol) Gives you grams of copper...
All atoms have the same mass?
All atoms do not have the same mass, but atoms of the same element generally do have the same mass.
How do you convert molar mass into moles?
to convert mass into moles you divide by the molar mass. You cannot convert molar mass directly to moles.
How do you convert mass into milligrams?
You can measure mass in milligrams but you do not convert mass into milligrams?
Why is the atomic mass unit rather then the gram usually used to express atomic mass?
Because a gram is too large a unit to express the mass of an atom. Also the atomic mass relates to what atoms or made of. If you convert the atomic mass into grams you have a mole of the element.
How do you convert grams to atoms with the avogadro's number?
(grams) x (6.02 x 10^23) / (mass number of specific element in grams)
Describe two changes due to the fusion of hydrogen?
This is a nuclear change. The atoms change into helium atoms. The loss of mass in the change convert to energy, thousands of times the amount of energy of a chemical change.
How do you convert 50.0 grams into atoms?
50 grams of whatever ( 1 mole of whatever/ mass of whatever)6.022 X 10^23/1 mole whatever) = so many atoms of whatever
Does mass and atoms form matter?
No. All mater on earth is made of atoms. Atoms have mass.
How many atoms are in 0.551 g of potassiom?
Seems to me we need to convert grams to atoms. So lets use mole conversion. When you convert from grams to moles, you divide the gram amount by the molar mass(a.k.a. atomic mass). So go look at a periodic table of elements and you'll see that pottasium's atomic mass is about 39.1 amu. So: .551/39.1 = .0141 mol Now that you have moles, you convert to particles(atoms). When converting from moles to anything you multiply…
Convert 3.55 grams of potassium to atoms?
Firstly, you'll need to convert the grams into number of mols. Since no. of mol = mass/molecular weight 3.55/39.1 = 0.09079 mol because 1 mol of potassium consists of 6.02x10^23 atoms; 0.09079 mol of potassium will consist of 0.09079x(6.02x10^23)=5.466x10^22 atoms of potassium.
How do you convert from moles to atoms and from mass to atoms?
moles to atoms you multiply the number of moles by avogadros number ex: 1.32 mol x (6.022 x 10^23 atoms)/mol mass to atoms you multiply the mass (in grams) times the molar mass of the element or compound (ex: N 14.01 mols/gram) then times avogadros number once you have the moles. ex: 45.6 g N x (14.01 mol/gram) x (6.022 x 10 ^23 atoms/mol) if it's a compound instead of an element, find the molar…
What is the mass of one mole atoms?
The mass depends on the element whose atoms are concerned. For eg, 1 mol Helium atoms have mass 4 g 1 mol Neon atoms have mass 20 g.
Atoms To Mass In Grams Converting Calculator
Is fusion nuclear decay or nuclear synthesis?
Fusion is nuclear synthesis, combining atoms of lesser mass into atoms of greater mass. Decay is reducing the mass of larger (unstable) atoms to form atoms of lesser mass.
Does mole of carbon atoms have a different mass than a mole of sulphur atoms?
Yes, the mass is different for each chemical element.
Is it true that not all kinds of matter have mass?
No. Everything has mass. Atoms have mass, Everything has atoms. :) Atomic Mass Number for example. :)
Atoms To Mass In Grams
How many Helium-atoms and Carbon-atoms do you have if you have He-atoms with mass of 40amu and C-atoms with mass of 120amu?
Mass of 1 Helium atom is 4 amu and mass of 1 Carbon atom is 12 amu. So there are 10 helium atoms and 10 carbon atoms

How do you convert atoms to grams with Avogadro's number?
divide the number of atoms by avogadros number (6.022*10^23), the resulting number is the number of moles you have. Multiply the number of moles of atoms by the molar mass (found on any periodic table) and the answer is how many grams of the substance you have.
How do you convert moles to grams and grams to moles?
MOLES TO GRAMS Multiply the number of moles by the molar mass of the substance. CONVERSION FACTOR Number of moles x Molar mass ////////////////////////// 1 mol GRAMS TO MOLES Divide grams by the molar mass of the substance. CONVERSION FACTOR Mass (g) x 1 mol /////////// molar mass Finding Molar Mass Number of Atoms Element A x Atomic Mass Element A (periodic table) = mass (g) Number of Atoms Element B x Atomic Mass Element…
What Make up 50 percent of the atomic mass of the nucleus?
Well, this is not exact, but smaller atoms have about the same number (and mass) of neutrons and of protons. Heavier atoms have a larger percentage of their mass in neutrons. Well, this is not exact, but smaller atoms have about the same number (and mass) of neutrons and of protons. Heavier atoms have a larger percentage of their mass in neutrons. Well, this is not exact, but smaller atoms have about the same number…
How can you convert volume to mass?
You can convert volume to mass by multiplying the volume by its density.
Mass To Atoms Calculator
How do you convert 1amu into grams?
The atomic mass unit (amu) is defined to be exactly 1/12 of the mass of a C-12 atom (Not a mole of carbon atoms...) Therefore 1 C-12 atom has a mass of exactly 12.00000000 amu. Also, therefore, 1 amu is a very small unit of mass - specifically 1amu = 1.66053X10^-24 grams. 1.00 mole of C-12 atoms has a mass of 12.0000000 grams!
Describe how you would convert a mass of CO2 to moles?
Moles To Atoms Calculator
To convert the mass of a carbon dioxide to moles takes two steps. First, sum the molecular weights of carbon and oxygen (oxygen's atomic weight is added twice because there are two oxygen atoms in the molecule). Then, simply divide the mass by the number calculated. The result is the number of moles of CO2
What is the mass number of most atoms atoms of oxygen?
What is the mass of 5.23x1021 platinum atoms?
Do atoms have mass and volume?
Yes, atoms have mass and volume. Any object that takes up space has mass and volume.
What has more mass 100 hydrogen atoms 4 sulfur atoms or 1 lanthanum atom?
100 Hydrogen atoms have an atomic mass of 100.794, 4 Sulfur atoms have an atomic mass of 128.26, and 1 Lanthanum atom has an atomic mass of 138.90547.
How do you convert from atoms to moles?
How Do You Convert Atoms To Mass In Grams
To convert atoms to moles you need to take the amount of atoms that you have, multiply it by 1 mol/ Avagadros number. That should give you the proper amount of moles.
Convert 1.53 x 1024 atoms of carbon to moles of carbon?
Convert 1.35 × 1024 atoms of carbon to moles of carbon
What is the mass in grams of 2x1012 atoms of potassium?
What is the mass, in grams, of 2 × 1012 atoms of potassium?